Multiple Choice
Picture (a) shows p-orbital overlap between carbon and oxygen atoms;picture (b) shows p-orbital overlap between silicon and oxygen atoms. 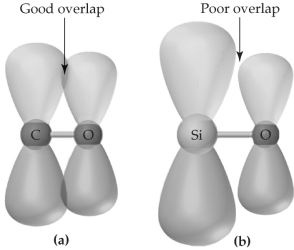
Based on these pictures,it is expected that
A) carbon and oxygen form better σ bonds than silicon and oxygen,so C-O single bonds are stronger than Si-O single bonds.
B) silicon and oxygen form better σ bonds than carbon and oxygen,so Si-O single bonds are stronger than C-O single bonds.
C) carbon and oxygen form better π bonds than silicon and oxygen,so C=O double bonds are stronger than Si=O double bonds.
D) silicon and oxygen form better π bonds than carbon and oxygen,so Si=O double bonds are stronger than C=O double bonds.
Correct Answer:

Verified
Correct Answer:
Verified
Q58: The net ionic reaction of solid CaH<sub>2</sub>
Q60: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which element shown
Q125: Which compound is not considered to be
Q127: In the following picture of an oxide,darkly-shaded
Q128: Which is classified as an ionic binary
Q129: In the following picture of an oxide,darkly-shaded
Q132: The following molecular orbital energy level diagram
Q134: In the picture representing binary hydride AH<sub>x</sub>,lightly-shaded
Q135: The high-temperature superconductor,YBa<sub>2</sub>Cu<sub>3</sub>O<sub>7</sub> can be prepared by
Q157: Water accounts for approximately two-thirds of the