Multiple Choice
The following molecular orbital energy level diagram shows the energies and occupancies of the MOs derived from the atomic 2p orbitals for an oxygen-containing binary compound of potassium.This compound is a 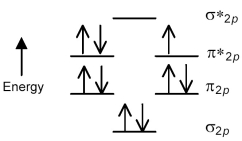
A) peroxide having a stronger O-O bond than O2.
B) peroxide having a weaker O-O bond than O2.
C) superoxide having a stronger O-O bond than O2.
D) superoxide having a weaker O-O bond than O2.
Correct Answer:

Verified
Correct Answer:
Verified
Q58: The net ionic reaction of solid CaH<sub>2</sub>
Q60: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which element shown
Q120: What is the chemical formula for the
Q124: Which reaction is not consistent with the
Q127: In the following picture of an oxide,darkly-shaded
Q128: Which is classified as an ionic binary
Q129: In the following picture of an oxide,darkly-shaded
Q130: Picture (a)shows p-orbital overlap between carbon and
Q134: In the picture representing binary hydride AH<sub>x</sub>,lightly-shaded
Q135: The high-temperature superconductor,YBa<sub>2</sub>Cu<sub>3</sub>O<sub>7</sub> can be prepared by