Multiple Choice
Look at the location of elements A,B,C,and D in the following periodic table.Consider the oxides that form when the elements A-D are in their highest oxidation states. 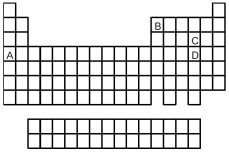
-Which oxide is the most basic?
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q29: An example of an isotope effect is
Q30: A binary compound that forms when potassium
Q31: The largest single industrial use of hydrogen
Q32: The oxygen-oxygen bond order in ozone,O<sub>3</sub>,is _.The
Q33: Hydrogen peroxide can act as an oxidizing
Q35: Look at the location of elements A,B,C,and
Q36: In the following picture of an oxide,darkly-shaded
Q37: At 25°C the dissociation constant K<sub>w</sub> for
Q38: Which of the following binary hydrides has
Q39: The following molecular orbital energy level diagram