Multiple Choice
The following molecular orbital energy level diagram shows the energies and occupancies of the MOs derived from the atomic 2p orbitals for an oxygen-containing binary compound of potassium.This compound is a 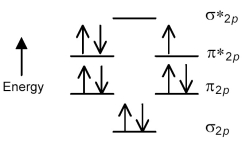
A) peroxide that is attracted by magnetic fields.
B) peroxide that is repelled by magnetic fields.
C) superoxide that is attracted by magnetic fields.
D) superoxide that is repelled by magnetic fields.
Correct Answer:

Verified
Correct Answer:
Verified
Q34: Look at the location of elements A,B,C,and
Q35: Look at the location of elements A,B,C,and
Q36: In the following picture of an oxide,darkly-shaded
Q37: At 25°C the dissociation constant K<sub>w</sub> for
Q38: Which of the following binary hydrides has
Q40: How many kilojoules of energy are released
Q41: Look at the location of elements A,B,C,and
Q42: At 25°C CO<sub>2</sub> is a gas and
Q43: The oxide of which element,identified by letters
Q44: What is the oxidation number of N