Multiple Choice
The following molecular orbital energy level diagram shows the energies and occupancies of the MOs derived from the atomic 2p orbitals for an oxygen-containing binary compound of potassium.This compound is a 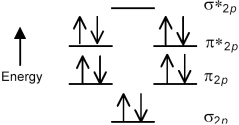
A) peroxide having a stronger O-O bond than O2.
B) peroxide having a weaker O-O bond than O2.
C) superoxide having a stronger O-O bond than O2.
D) superoxide having a weaker O-O bond than O2.
Correct Answer:

Verified
Correct Answer:
Verified
Q41: Look at the location of elements A,B,C,and
Q42: At 25°C CO<sub>2</sub> is a gas and
Q43: The oxide of which element,identified by letters
Q44: What is the oxidation number of N
Q45: Indicate the coefficient in front of H<sub>2</sub>O<sub>2</sub>
Q48: What are the four major ionic constituents
Q49: How many grams of water are required
Q51: A reaction occurs when 0.2105 g of
Q83: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which element shown
Q287: In which compound does oxygen have a