Multiple Choice
Look at the location of elements A,B,C,and D in the following periodic table.The oxide of which element in its highest oxidation state can react with both H+(aq) and OH-(aq) ? 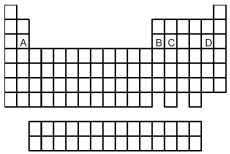
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q36: In the following picture of an oxide,darkly-shaded
Q37: At 25°C the dissociation constant K<sub>w</sub> for
Q38: Which of the following binary hydrides has
Q39: The following molecular orbital energy level diagram
Q40: How many kilojoules of energy are released
Q42: At 25°C CO<sub>2</sub> is a gas and
Q43: The oxide of which element,identified by letters
Q44: What is the oxidation number of N
Q45: Indicate the coefficient in front of H<sub>2</sub>O<sub>2</sub>
Q46: The following molecular orbital energy level diagram