Multiple Choice
The third transition series metals are shown below. 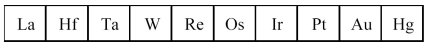
-Which has the highest melting point?
A) La
B) W
C) Os
D) Hg
Correct Answer:

Verified
Correct Answer:
Verified
Q1: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which element indicated
Q2: What is the correct formula for tetraamminecarbonatoiron(III)chloride?<br>A)(NH<sub>3</sub>)<sub>4</sub>[FeCO<sub>3</sub>]Cl<br>B)[Fe(CO<sub>3</sub>)(NH<sub>3</sub>)<sub>4</sub>]Cl<br>C)[Fe(CO<sub>3</sub>)(NH<sub>3</sub>)<sub>4</sub>]Cl<sub>2</sub><br>D)[Fe(CO<sub>3</sub>)Cl(NH<sub>3</sub>)<sub>4</sub>]
Q3: The number of unpaired electrons in square
Q4: What statement is most inconsistent about the
Q6: What chemical equation represents the best method
Q8: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which element indicated
Q9: What is a representative orbital-filling diagram for
Q10: The second transition series metals are shown
Q11: The first transition series metals are shown
Q100: What is the ground-state electron configuration for