Multiple Choice
Each of the following containers illustrates a solution in which the black spheres represent solute. 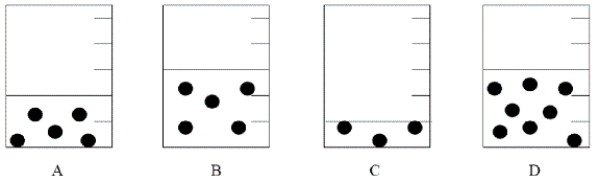 Which is the most concentrated solution?
Which is the most concentrated solution?
A) A
B) All have the same concentration.
C) B
D) C
E) D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q145: An excess of sodium sulfate was added
Q146: What is the net ionic equation for
Q147: What volume of 1.08 M HCl is
Q148: Which of the following reactions best describes
Q149: A 60.00-mL sample of a weak acid
Q151: The sum of all the oxidation numbers
Q152: All of the following reactions can be
Q153: What is the balanced oxidation half-reaction provided
Q154: What is the molarity of hydrochloric acid
Q155: Which of the following is a weak