Multiple Choice
An excess of sodium sulfate was added to a 1.000-L sample of polluted water.The mass of lead (II) sulfate that precipitated was 308.88 mg.Determine the mass of lead present in the sample of water.
Na2SO4(aq) + Pb(NO3) 2(aq) 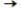
2NaNO3(aq) + PbSO4(s)
A) 108.15 mg
B) 88.02 mg
C) 180.08 mg
D) 145.56 mg
E) 211.03 mg
Correct Answer:

Verified
Correct Answer:
Verified
Q140: A student must prepare 7.00 L of
Q141: Which of the following is best described
Q142: When solutions of barium iodide and lithium
Q143: _ is a type of quantitative analysis
Q144: Identify the spectator ion(s)in the following reaction.<br>Zn(OH)<sub>2</sub>(s)+
Q146: What is the net ionic equation for
Q147: What volume of 1.08 M HCl is
Q148: Which of the following reactions best describes
Q149: A 60.00-mL sample of a weak acid
Q150: Each of the following containers illustrates a