Multiple Choice
When the valve between the 2.00-L bulb,in which the gas pressure is 2.50 atm,and the 3.00-L bulb,in which the gas pressure is 1.50 atm,is opened,what will be the final pressure in the two bulbs? Assume the temperature remains constant. 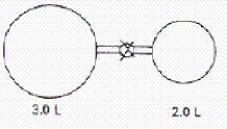
A) 1.90 atm
B) 4.00 atm
C) 2.17 atm
D) 2.10 atm
E) 1.83 atm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q85: A sample of hydrogen was collected by
Q86: If 636.0 mL of nitrogen gas,measured at
Q87: A fixed amount of gas in a
Q88: A gas occupies a volume of 2.00
Q89: A 1.00-L bulb contains a sample of
Q91: A sample of oxygen is collected over
Q92: How many moles of gas are in
Q93: A 22.4 L high pressure reaction vessel
Q94: Which of the following is included as
Q95: A gas occupies a volume of 2.75