Multiple Choice
A 1.00-L bulb contains a sample of O2 at 25°C and 1.00 atm pressure.A second 1.00-L bulb contains a sample of CH4 at 25°C and 1.00 atm pressure.What is the ratio of the number of molecules of methane to the number of molecules of oxygen in each of the containers?
A) 2:5
B) 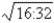
C) 5:2
D) 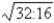
E) 1:1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q84: Calculate the root-mean-square velocity for the O<sub>2</sub>
Q85: A sample of hydrogen was collected by
Q86: If 636.0 mL of nitrogen gas,measured at
Q87: A fixed amount of gas in a
Q88: A gas occupies a volume of 2.00
Q90: When the valve between the 2.00-L bulb,in
Q91: A sample of oxygen is collected over
Q92: How many moles of gas are in
Q93: A 22.4 L high pressure reaction vessel
Q94: Which of the following is included as