Multiple Choice
Given: 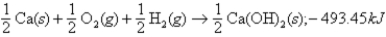 what is ΔH for the following thermochemical equation?
what is ΔH for the following thermochemical equation? 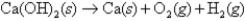
A) 986.9 kJ
B) -986.9 kJ
C) -139 MJ
D) -2320 kJ
E) -38.7 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q100: Consider the following thermochemical equation:<br>N<sub>2</sub>(g)+ 2O<sub>2</sub>(g)→ 2NO<sub>2</sub>(g);
Q101: A bomb calorimeter has a heat capacity
Q102: Which of the following is not a
Q103: Which of the following is an endothermic
Q104: A 86.9-g sample of chromium (s =
Q106: Which of the following sentences accurately describes
Q107: How much heat is released at constant
Q108: Which of the following statements is true
Q109: The energy associated with an object held
Q110: A 3.540-g sample of an unknown metal