Multiple Choice
A bomb calorimeter has a heat capacity of 2.47 kJ/K.When a 0.106-g sample of a certain hydrocarbon was burned in this calorimeter,the temperature increased by 2.14 K.Calculate the energy of combustion for 1 g of the hydrocarbon.
A) -5.29 J/g
B) 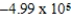 J/g
J/g
C) -0.120 J/g
D) 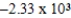 J/g
J/g
E) -0.560 J/g
Correct Answer:

Verified
Correct Answer:
Verified
Q96: The energy associated with the motion of
Q97: The quantity of heat required to raise
Q98: Given:<br>Pb(s)+ PbO<sub>2</sub>(s)+ 2H<sub>2</sub>SO<sub>4</sub>(l)→ 2PbSO<sub>4</sub>(s)+ 2H<sub>2</sub>O(l); ΔH° =
Q99: The standard enthalpies of formation of various
Q100: Consider the following thermochemical equation:<br>N<sub>2</sub>(g)+ 2O<sub>2</sub>(g)→ 2NO<sub>2</sub>(g);
Q102: Which of the following is not a
Q103: Which of the following is an endothermic
Q104: A 86.9-g sample of chromium (s =
Q105: Given: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Given: what
Q106: Which of the following sentences accurately describes