Multiple Choice
What is the quantity of heat evolved at constant pressure when 30.5 g H2O(l) is formed from the combustion of H2(g) and O2(g) ?
H2(g) + 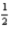
O2(g) → H2O(l) ; ΔH° = -285.8 kJ
A) 5.92 × 10-3 kJ
B) 285.8 kJ
C) 8.72 × 103 kJ
D) 168 kJ
E) 4.84 × 102 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q70: Which of the following statements is incorrect
Q71: What is the change in enthalpy when
Q72: The molar heat capacity of gaseous heptane
Q73: How much heat is gained by copper
Q74: Which of the following reactions corresponds to
Q76: A 110.0-g sample of metal at 82.00°C
Q77: What mass of oxygen is consumed when
Q78: Which of the following does not result
Q79: The heat required to raise the temperature
Q80: Which of the following has a standard