Multiple Choice
What mass of oxygen is consumed when 285.5 kJ of energy is evolved from the combustion of a mixture of H2(g) and O2(g) ?
H2(g) + 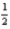
O2(g) → H2O(l) ; ΔH° = -285.8 kJ
A) 15.98 g
B) 31.96 g
C) 0.01564 g
D) 31.98 g
E) 0.01679 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q72: The molar heat capacity of gaseous heptane
Q73: How much heat is gained by copper
Q74: Which of the following reactions corresponds to
Q75: What is the quantity of heat evolved
Q76: A 110.0-g sample of metal at 82.00°C
Q78: Which of the following does not result
Q79: The heat required to raise the temperature
Q80: Which of the following has a standard
Q81: What is the standard enthalpy of formation
Q82: Which of the following statements is not