Multiple Choice
What quantity,in moles,of oxygen is consumed when 717.4 kJ of energy is evolved from the combustion of a mixture of H2(g) and O2(g) ?
H2(g) + 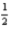
O2(g) → H2O(l) ; ΔH° = -285.8 kJ
A) 1.255 mol
B) 2.519482 mol
C) 0.1991 mol
D) 1.755 mol
E) 0.7559321 mol
Correct Answer:

Verified
Correct Answer:
Verified
Q86: What is the standard enthalpy change for
Q87: Using two or more of the following,<br>N<sub>2</sub>(g)+
Q88: The internal energy of a substance is
Q89: H<sub>2</sub> and F<sub>2</sub> react according to the
Q90: Under conditions of constant pressure,for which of
Q92: At constant pressure,the sign of q for
Q93: A 70.4-L sample of a gaseous hydrocarbon,measured
Q94: At 25°C,when 1.00 g of sulfur is
Q95: When 22.0 mL of liquid benzene (C<sub>6</sub>H<sub>6</sub>,d
Q96: The energy associated with the motion of