Multiple Choice
Using two or more of the following,
N2(g) + 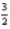
O2(g) → N2O3(s) ; ΔH° = 83.7 kJ
N2(g) + O2(g) → 2NO(g) ; ΔH° = 180.4 kJ 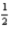
N2(g) + O2(g) → NO2(g) ; ΔH° = 33.2 kJ 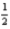
N2(g) + 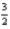
H2(g) → NH3(g) ; ΔH° = −45.9 kJ
Determine ΔH° for the following reaction.
NO(g) + NO2(g) → N2O3(g)
A) -39.7 kJ
B) 24.3 kJ
C) -207.1 kJ
D) 39.7 kJ
E) 207.1 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q82: Which of the following statements is not
Q83: The phrase "the heat absorbed or released
Q84: What is the standard enthalpy of formation
Q85: A 8.22-g sample of solid calcium reacted
Q86: What is the standard enthalpy change for
Q88: The internal energy of a substance is
Q89: H<sub>2</sub> and F<sub>2</sub> react according to the
Q90: Under conditions of constant pressure,for which of
Q91: What quantity,in moles,of oxygen is consumed when
Q92: At constant pressure,the sign of q for