Multiple Choice
What is the energy of a photon of electromagnetic radiation with a frequency of 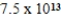 Hz? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)
Hz? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)
A) 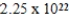 J
J
B) 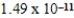 J
J
C) 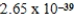 J
J
D) 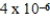 J
J
E) 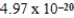 J
J
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q58: Which type of electromagnetic radiation has the
Q59: What is the total number of subshells
Q60: When an electron in an atom makes
Q61: When a particular metal is illuminated with
Q62: The number of orbitals having a given
Q63: A possible value of the magnetic quantum
Q64: The electron in a hydrogen atom,originally in
Q66: The branch of physics that mathematically describes
Q67: Consider the following energy-level diagram for a
Q68: Which of the following statements is incorrect