Multiple Choice
Consider the following energy-level diagram for a particular electron in an atom. 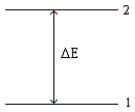 Based on this diagram,which of the following statements is incorrect?
Based on this diagram,which of the following statements is incorrect?
A) The wavelength of a photon emitted by the electron jumping from level 2 to level 1 is given by 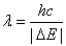 .
.
B) If the electron is in level 1,it may jump to level 2 by absorbing a photon with energy of ΔE.
C) If the electron is in level 1,it may jump to level 2 by absorbing any photon having energy of at least ΔE.
D) We would observe an electron jumping from level 2 to level 1 as a single line in a line spectrum.
E) If the electron is in level 2,it may jump to level 1 by emitting a photon with energy of |ΔE|.
Correct Answer:

Verified
Correct Answer:
Verified
Q58: Which type of electromagnetic radiation has the
Q59: What is the total number of subshells
Q60: When an electron in an atom makes
Q61: When a particular metal is illuminated with
Q62: The number of orbitals having a given
Q63: A possible value of the magnetic quantum
Q64: The electron in a hydrogen atom,originally in
Q65: What is the energy of a photon
Q66: The branch of physics that mathematically describes
Q68: Which of the following statements is incorrect