Multiple Choice
From a consideration of the phase diagram below,a change from point M to point N corresponds to 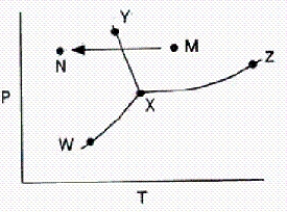
A) sublimation.
B) liquefaction.
C) evaporation.
D) condensation.
E) freezing.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: What is the maximum number of hydrogen
Q3: Knowing that ΔH<sub>vap</sub> for water is 40.7
Q4: Sulfur trioxide (SO<sub>3</sub>)is able to be liquefied
Q5: What is the enthalpy of vaporization of
Q6: Which of the following pure substances has
Q7: Lithium chloride crystallizes in a face-centered cubic
Q8: How many atoms are there in a
Q9: Which of the following is not a
Q10: Which of the following pure substances has
Q11: A low melting solid readily dissolves in