Multiple Choice
Consider the following gas-aqueous liquid equilibrium for a closed system at a constant temperature.
O2(g) 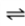 O2(aq)
O2(aq)
What is the effect on the equilibrium composition of the liquid when the partial pressure of O2 gas above the liquid is decreased?
A) The amount of O2 dissolved in the liquid decreases.
B) The amount of O2 dissolved in the liquid increases.
C) The amount of O2 dissolved in the liquid does not change.
D) Not enough information is provided to answer the question.
E) Either A or B.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: To determine the molar mass of a
Q3: If the solubility of O<sub>2</sub> at 0.160
Q4: A solution consisting of 0.276 mol of
Q5: Which of the following sets of conditions
Q6: Benzene,C<sub>6</sub>H<sub>6</sub>,and toluene,C<sub>6</sub>H<sub>5</sub>CH<sub>3</sub>,form ideal solutions.At 35°C the vapor
Q7: Which of the following sets of conditions
Q8: A red blood cell placed in pure
Q9: When 1 mol of a nonvolatile nonelectrolyte
Q10: Which of the following statements best describes
Q11: What is the mole fraction of urea