Multiple Choice
For the hypothetical reaction A → products,the concentration of A was monitored over time.From the following graph,what is the rate constant for the decomposition of A? 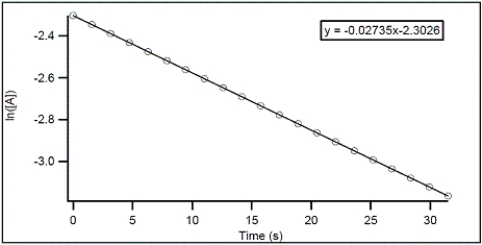
A) -0.02735 s-1
B) 2.3026 s-1
C) -2.3026 s-1
D) 0.02735 s-1
E) 0.01188 s-1
Correct Answer:

Verified
Correct Answer:
Verified
Q103: Which of the following is not a
Q104: At a given temperature,a first-order reaction has
Q105: For the first-order reaction<br>1/2 N<sub>2</sub>O<sub>4</sub>(g)→ NO<sub>2</sub>(g); ΔH
Q106: The half-life of a reaction is<br>A)twice as
Q107: Which of the following reactions is not
Q108: For the hypothetical reaction A → products,the
Q110: Which of the following corresponds to the
Q111: In the reaction 2H<sub>2</sub>O<sub>2</sub>(aq)→ 2H<sub>2</sub>O(l)+ O<sub>2</sub>(g),the initial
Q112: The decomposition of ozone may occur through
Q113: For a certain first-order reaction with the