Multiple Choice
Which expression correctly describes the equilibrium constant Kc for the following reaction?
2C2H2(g) + 5O2(g) 
4CO2(g) + 2H2O(g)
A) 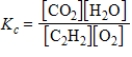
B) 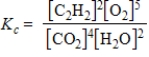
C) 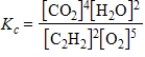
D) 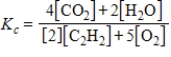
E) 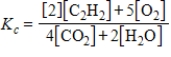
Correct Answer:

Verified
Correct Answer:
Verified
Q12: What is the balanced equation for the
Q13: For which of the following values of
Q14: Consider the following system at equilibrium: N<sub>2</sub>(g)+
Q15: The reaction quotient for a system is
Q16: Consider the reaction<br>S<sub>2</sub>Cl<sub>2</sub>(l)+ CCl<sub>4</sub>(l) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Consider
Q18: Consider the following equilibrium:<br>1/2N<sub>2</sub>O<sub>4</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Consider
Q19: At 298 K,the value of K<sub>c</sub> for
Q20: A(n)_ is a substance that increases the
Q21: For which of the following equilibria does
Q22: Given the equilibrium constants for the following