Multiple Choice
The reaction quotient for a system is 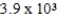 .If the equilibrium constant for the system is
.If the equilibrium constant for the system is 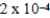
,what will happen as the reaction mixture approaches equilibrium?
A) The equilibrium constant will increase until it equals the reaction quotient.
B) There will be a net gain in both product(s) and reactant(s) .
C) There will be a net gain in product(s) .
D) There will be a net gain in reactant(s) .
E) The equilibrium constant will decrease until it equals the reaction quotient.
Correct Answer:

Verified
Correct Answer:
Verified
Q10: Solid HgO,liquid Hg,and gaseous O<sub>2</sub> are placed
Q11: Which of the following statements is true
Q12: What is the balanced equation for the
Q13: For which of the following values of
Q14: Consider the following system at equilibrium: N<sub>2</sub>(g)+
Q16: Consider the reaction<br>S<sub>2</sub>Cl<sub>2</sub>(l)+ CCl<sub>4</sub>(l) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Consider
Q17: Which expression correctly describes the equilibrium constant
Q18: Consider the following equilibrium:<br>1/2N<sub>2</sub>O<sub>4</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Consider
Q19: At 298 K,the value of K<sub>c</sub> for
Q20: A(n)_ is a substance that increases the