Multiple Choice
What is the equilibrium pH of an initially 0.73 M solution of the monoprotic acid benzoic acid at 25°C (Ka = 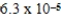 ) ?
) ?
A) 2.17
B) 7.00
C) 1.87
D) 12.13
E) 5.13
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: What is the hydronium-ion concentration at equilibrium
Q19: In a 0.01 M solution of 1,4-butanedicarboxylic
Q20: What is the pH of an initially
Q21: What is the equilibrium concentration of H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>
Q22: Which of the following salts will produce
Q24: Consider the reaction NH<sub>3</sub>(aq)+ H<sub>2</sub>O(l) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg"
Q25: The equilibrium hydronium ion concentration of an
Q26: What is the percent ionization of a
Q27: A 0.10 M solution of a weak
Q28: For the equilibrium that exists in an