Multiple Choice
The equilibrium hydronium ion concentration of an initially 0.510 M solution of a diprotic weak acid (H2A) is 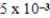 M.What is Ka1? (assume Ca/Ka ≥ 102 and Ka1 >> Ka2)
M.What is Ka1? (assume Ca/Ka ≥ 102 and Ka1 >> Ka2)
A) 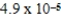
B) 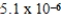
C) 
D) 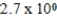
E) not enough information provided
Correct Answer:

Verified
Correct Answer:
Verified
Q20: What is the pH of an initially
Q21: What is the equilibrium concentration of H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>
Q22: Which of the following salts will produce
Q23: What is the equilibrium pH of an
Q24: Consider the reaction NH<sub>3</sub>(aq)+ H<sub>2</sub>O(l) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg"
Q26: What is the percent ionization of a
Q27: A 0.10 M solution of a weak
Q28: For the equilibrium that exists in an
Q29: Which of the following salts will produce
Q30: A 0.010 M aqueous solution of a