Multiple Choice
Which of the following salts is most likely to form an aqueous solution having the pH shown in the figure below? 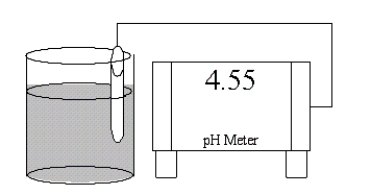
A) NH4Cl
B) NaBr
C) K2CO3
D) RbCN
E) LiNO3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: What is the hydroxide-ion concentration of a
Q2: Calculate the pH of a solution that
Q3: What is K<sub>a</sub> for the anilinium cation,C<sub>6</sub>H<sub>5</sub>NH<sub>3</sub><sup>+</sup>,at
Q4: What is the hydronium-ion concentration in a
Q5: For a 0.10 M solution of glutaric
Q7: Saccharin is a weak organic base with
Q8: Which of the following solutions will not
Q9: Which acid-base combination is depicted by this
Q10: What will happen if a small amount
Q11: Suppose a buffer solution is made from