Multiple Choice
Which acid-base combination is depicted by this titration curve? 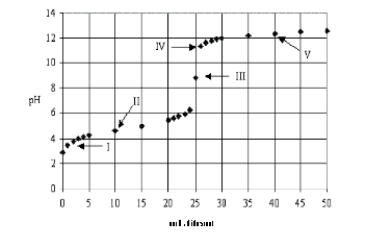
A) Titration of a weak base with a strong acid.
B) Titration of a weak acid with a strong base.
C) Titration of a strong acid with a strong base.
D) Titration of a strong base with a strong acid.
E) Not enough information provided.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: What is the hydronium-ion concentration in a
Q5: For a 0.10 M solution of glutaric
Q6: Which of the following salts is most
Q7: Saccharin is a weak organic base with
Q8: Which of the following solutions will not
Q10: What will happen if a small amount
Q11: Suppose a buffer solution is made from
Q12: Which of the following solutions would show
Q13: What is the pH of a 0.35
Q14: Which of the following salts is most