Multiple Choice
What is the equilibrium fluoride ion concentration of a solution that initially consists of 0.23 M HF and 0.400 M HCl? Ka for HF is 6.8 × 10-4.
A) 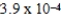 M
M
B)  M
M
C) 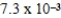 M
M
D) 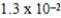 M
M
E) 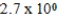 M
M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q99: A 8.0 × 10<sup>-3</sup> M solution of
Q100: What is the pOH of a 0.17
Q101: Equal moles of the indicated acids are
Q102: What is the pH of a solution
Q103: A weak acid,HF,is in solution with dissolved
Q105: The two acid-ionization constants for sulfurous acid,H<sub>2</sub>SO<sub>3</sub>,are
Q106: The amphiprotic anion monohydrogenphosphate acts as both
Q107: What is the pH at the equivalence
Q108: What is the pH of a solution
Q109: What is the hydronium-ion concentration of a