Multiple Choice
Equal moles of the indicated acids are dissolved in the amounts of water shown in the beakers below.In which solution will the percent ionization of the acid be the lowest? 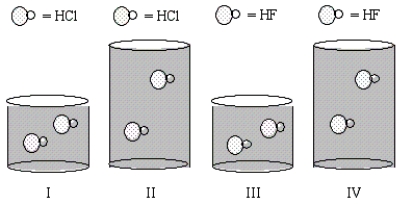
A) All have equal percent ionization of acid.
B) III
C) IV
D) II
E) I
Correct Answer:

Verified
Correct Answer:
Verified
Q96: A 8.42-g sample of homogentisic acid,a weak
Q97: Which of the following salts is most
Q98: In the titration of a weak monoprotic
Q99: A 8.0 × 10<sup>-3</sup> M solution of
Q100: What is the pOH of a 0.17
Q102: What is the pH of a solution
Q103: A weak acid,HF,is in solution with dissolved
Q104: What is the equilibrium fluoride ion concentration
Q105: The two acid-ionization constants for sulfurous acid,H<sub>2</sub>SO<sub>3</sub>,are
Q106: The amphiprotic anion monohydrogenphosphate acts as both