Multiple Choice
The following titration curve depicts the titration of a weak base with a strong acid.Which of the labeled points is the equivalence point. 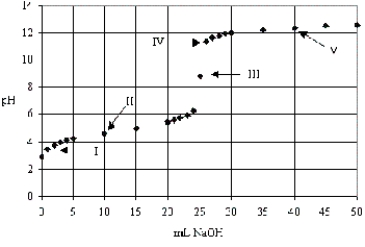
A) III only
B) II only
C) I only
D) V only
E) IV only
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q88: A 75.0-mL sample of 0.0500 M HCN
Q89: What molar ratio of acetic acid to
Q90: What will happen if a small amount
Q91: What is the hydroxide-ion concentration of a
Q92: What is the pH of the solution
Q94: A solution of aniline (C<sub>6</sub>H<sub>5</sub>NH<sub>2</sub>,K<sub>b</sub> = 4.2
Q95: What is the hydroxide-ion concentration in a
Q96: A 8.42-g sample of homogentisic acid,a weak
Q97: Which of the following salts is most
Q98: In the titration of a weak monoprotic