Multiple Choice
Which of the following salts is most likely to form an aqueous solution having the pH shown in the figure below? 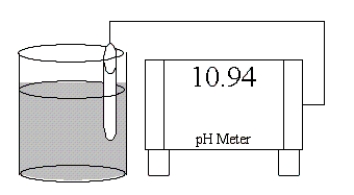
A) KCl
B) Zn(NO3) 2
C) NaCN
D) NH4Cl
E) LiBr
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q92: What is the pH of the solution
Q93: The following titration curve depicts the titration
Q94: A solution of aniline (C<sub>6</sub>H<sub>5</sub>NH<sub>2</sub>,K<sub>b</sub> = 4.2
Q95: What is the hydroxide-ion concentration in a
Q96: A 8.42-g sample of homogentisic acid,a weak
Q98: In the titration of a weak monoprotic
Q99: A 8.0 × 10<sup>-3</sup> M solution of
Q100: What is the pOH of a 0.17
Q101: Equal moles of the indicated acids are
Q102: What is the pH of a solution