Multiple Choice
The silver-ion concentration in a saturated solution of silver(I) sulfate is 2.9 × 10-2 M.What is Ksp for silver(I) sulfate?
A) 6.9 × 10-7
B) 2.1 × 10-4
C) 9.6 × 10-5
D) 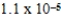
E) 8.3 × 10-4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q92: Cyanide ion forms very stable complex ions
Q93: _ involves the determination of the identity
Q94: What is the solubility (in g/L)of calcium
Q95: For which of the following will precipitation
Q96: Rank the following metal sulfides in order
Q98: What is the best way to ensure
Q99: Calculate the molar concentration of uncomplexed Zn<sup>2+</sup>(aq)in
Q100: Which of the following particulate views is/are
Q101: Suppose 50.00 mL of 2.0 × 10<sup>-6</sup>
Q102: The best explanation for the dissolution of