Multiple Choice
Cyanide ion forms very stable complex ions with a variety of metal ions.What is the molar equilibrium concentration of uncomplexed Ni2+(aq) in a solution that initially contains 1.3 mol of Ni(CN) 42− per liter of solution .Kf for Ni(CN) 42− is  .
.
A)  M
M
B) 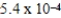 M
M
C) 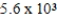 M
M
D) 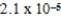 M
M
E) 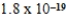 M
M
Correct Answer:

Verified
Correct Answer:
Verified
Q87: Sodium chloride is added slowly to a
Q88: Which Figures I-IV represent(s)the result of mixing
Q89: What is the relationship between molar solubility
Q90: What is the pH of a saturated
Q91: What will happen if 0.1 mol of
Q93: _ involves the determination of the identity
Q94: What is the solubility (in g/L)of calcium
Q95: For which of the following will precipitation
Q96: Rank the following metal sulfides in order
Q97: The silver-ion concentration in a saturated solution