Multiple Choice
Which Figures I-IV represent(s) the result of mixing aqueous solutions of NaOH and CuCl2 in which the ion product Qc > Ksp for the insoluble product? (C = cation,A = anion) 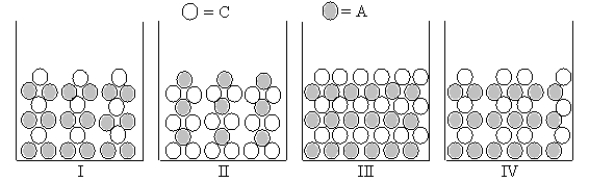
A) only II
B) both I and II
C) only IV
D) only I
E) only III
Correct Answer:

Verified
Correct Answer:
Verified
Q83: The insoluble salts AV,B<sub>2</sub>W,C<sub>2</sub>X<sub>3</sub>,DY<sub>2</sub>,and EZ<sub>3</sub>,which were formed
Q84: Suppose 50.00 mL of a 1 ×
Q85: If 275 mL of 1 × 10<sup>-7</sup>
Q86: What is the maximum hydroxide-ion concentration that
Q87: Sodium chloride is added slowly to a
Q89: What is the relationship between molar solubility
Q90: What is the pH of a saturated
Q91: What will happen if 0.1 mol of
Q92: Cyanide ion forms very stable complex ions
Q93: _ involves the determination of the identity