Multiple Choice
If the cell is initially at standard-state conditions,which of the following statements is true? 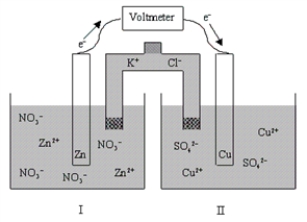 Zn2+(aq) + 2e-
Zn2+(aq) + 2e-  Zn(s) ; E° = -0.76 V
Zn(s) ; E° = -0.76 V
Cu2+(aq) + 2e-  Cu(s) ; E° = 0.34 V
Cu(s) ; E° = 0.34 V
A) Initially 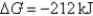 ,and it will become more positive with time.
,and it will become more positive with time.
B) Initially 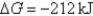 ,and it will not change with time.
,and it will not change with time.
C) Initially 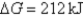 ,and it will become more negative with time.
,and it will become more negative with time.
D) Initially 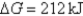 ,and it will become more positive with time.
,and it will become more positive with time.
E) Initially 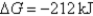 ,and it will become more negative with time.
,and it will become more negative with time.
Correct Answer:

Verified
Correct Answer:
Verified
Q48: The electrochemical reaction which powers a lead-acid
Q49: Given: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Given:
Q50: Electrolysis of a molten salt with the
Q51: Given:<br>Zn<sup>2+</sup>(aq)+ 2e<sup>-</sup> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Given: Zn<sup>2+</sup>(aq)+ 2e<sup>-</sup>
Q52: In the electrolysis of an acid solution,oxygen
Q54: How many faradays are required to convert
Q55: Consider the following standard reduction potentials:<br>Mg<sup>2+</sup>(aq)+ 2e<sup>-</sup>
Q56: The anode in a voltaic cell and
Q57: A current of 13.0 A is passed
Q58: Balance the following half-reaction occurring in basic