Multiple Choice
Given: 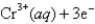

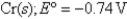
Pb2+ (aq) + 2e¯  Pb(s) ; Eº = -0.13V
Pb(s) ; Eº = -0.13V
What is the standard Gibbs free-energy change for the following reaction?
2Cr(s) + 3Pb2+ (aq) → 3Pb(s) + 2Cr3+ (aq)
A) 353kJ
B) 118kJ
C) 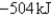
D) -353kJ
E) 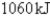
Correct Answer:

Verified
Correct Answer:
Verified
Q44: What is the value of the reaction
Q45: A cell consists of a magnesium electrode
Q46: For the cell reaction<br>2MnO<sub>4</sub><sup>-</sup> (aq)+ 5SO<sub>2</sub>(g)+ 2H<sub>2</sub>O(l)<sup>
Q47: A zinc-copper voltaic cell is represented as
Q48: The electrochemical reaction which powers a lead-acid
Q50: Electrolysis of a molten salt with the
Q51: Given:<br>Zn<sup>2+</sup>(aq)+ 2e<sup>-</sup> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Given: Zn<sup>2+</sup>(aq)+ 2e<sup>-</sup>
Q52: In the electrolysis of an acid solution,oxygen
Q53: If the cell is initially at standard-state
Q54: How many faradays are required to convert