Multiple Choice
A solution contains 12.5% NaCl by mass.What mass of solution is required to obtain 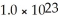 Na atoms?
Na atoms?
A) 0.013 g
B) 30 g
C) 1.2 g
D) 95 g
E) 78 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q74: What is the isotopic atomic mass of
Q75: J.J.Thomson suggested the "plum pudding" model of
Q76: The total numbers of neutrons,protons,and electrons in
Q77: A certain mass of nickel reacts with
Q78: The natural abundance of calcium in the
Q80: Isotopes have different atomic number (Z)but the
Q81: The atomic mass interval for carbon is
Q82: What is the average mass in kilograms
Q83: Calculate the percent abundance of the two
Q84: How many atoms of lead are required