Multiple Choice
Calculate the percent abundance of the two isotopes of a fictional element,georgium,if it has an average atomic mass of 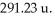
Go-290 289.86 u
Go-292 292.07 u
A) Go-290,38% and Go-292,62%
B) Go-290,62% and Go-292,38%
C) Go-290,42% and Go-292,58%
D) Go-290,58% and Go-292,42%
E) cannot be determined
Correct Answer:

Verified
Correct Answer:
Verified
Q78: The natural abundance of calcium in the
Q79: A solution contains 12.5% NaCl by mass.What
Q80: Isotopes have different atomic number (Z)but the
Q81: The atomic mass interval for carbon is
Q82: What is the average mass in kilograms
Q84: How many atoms of lead are required
Q85: The following ratios of masses were obtained
Q86: Choose the correct statement.<br>A)Neutrons have no charge
Q87: What is the density (g/cm<sup>3</sup>)of tin if
Q88: Lead-206 has a relative abundance of 23.6%.What