Multiple Choice
Lead-206 has a relative abundance of 23.6%.What size block of lead (in cubic centimeters) will contain 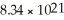
Atoms of lead-206? The density of lead is 11.35 g/cm3.
A) 0.0107 cm3
B) 0.253 cm3
C) 1.07 cm3
D) 0.330 cm3
E) 12.1 cm3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q83: Calculate the percent abundance of the two
Q84: How many atoms of lead are required
Q85: The following ratios of masses were obtained
Q86: Choose the correct statement.<br>A)Neutrons have no charge
Q87: What is the density (g/cm<sup>3</sup>)of tin if
Q89: Which of the following statements is true
Q90: Write the symbol for the most common
Q91: Rubidium possesses two stable forms and has
Q92: Which of the following is a correct
Q93: Choose the INCORRECT statement.<br>A)Gamma rays are bent