Multiple Choice
A stock solution of 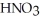 Is prepared and found to contain 13.5 mol L-1 of
Is prepared and found to contain 13.5 mol L-1 of 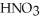 (aq) .If 25.0 mL of the stock solution is diluted with water to a final volume of 0.500 L,what is the concentration of the diluted solution?
(aq) .If 25.0 mL of the stock solution is diluted with water to a final volume of 0.500 L,what is the concentration of the diluted solution?
A) 0.270 mol L-1
B) 1.48 mol L-1
C) 0.675 mol L-1
D) 675 mol L-1
E) 270 mol L-1
Correct Answer:

Verified
Correct Answer:
Verified
Q68: Given the reaction:<br>2KMnO<sub>4</sub> + 10 KI +
Q69: What is the molarity of methanol, <img
Q70: What is the sum of the coefficients
Q71: How many grams of CH<sub>3</sub>OH must
Q72: What are the smallest,whole-number coefficients in front
Q74: The molarity of a solution that contains
Q75: What is the molarity of formaldehyde in
Q76: What is the percent yield if 122
Q77: If 0.500 mol of CaCl<sub>2</sub> is mixed
Q78: Cryolite is a compound needed for the