Multiple Choice
What is the molarity of methanol, 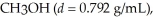
If 150.0 mL is dissolved in enough water to make 4.00 L of solution?
A) 3.71 M
B) 1.17 M
C) 1.48 M
D) 0.927 M
E) 0.734 M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q64: Identify the coefficient of NO(g)when the following
Q65: A student prepared a stock solution by
Q66: The Haber Process for the production of
Q67: Lithium and nitrogen react in a combination
Q68: Given the reaction:<br>2KMnO<sub>4</sub> + 10 KI +
Q70: What is the sum of the coefficients
Q71: How many grams of CH<sub>3</sub>OH must
Q72: What are the smallest,whole-number coefficients in front
Q73: A stock solution of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="A
Q74: The molarity of a solution that contains