Multiple Choice
Identify the coefficient of NO(g) when the following reaction is balanced,and calculate the volume of nitrogen monoxide gas produced when 8.00 g of ammonia is reacted with 12.0 g of oxygen at 25 °C.The density of nitrogen monoxide at 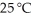
Is 1.23 g L-1.
(unbalanced) NH3(g) + O2(g) → NO(g) + H2O(l)
A) coefficient 4,volume 7.32 L
B) coefficient 1,volume 11.1 L
C) coefficient 2,volume 11.5 L
D) coefficient 3,volume 17.3 L
Correct Answer:

Verified
Correct Answer:
Verified
Q59: What mass of trisodium phosphate is required
Q60: How many moles of BCl<sub>3</sub> are needed
Q61: A formula is a shorthand way of
Q62: How many grams of AgNO<sub>3</sub> are needed
Q63: What volume of concentrated acetic acid <img
Q65: A student prepared a stock solution by
Q66: The Haber Process for the production of
Q67: Lithium and nitrogen react in a combination
Q68: Given the reaction:<br>2KMnO<sub>4</sub> + 10 KI +
Q69: What is the molarity of methanol, <img