Multiple Choice
In the reaction 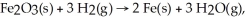
How many moles of iron can be produced using 17.4 liters of hydrogen at STP?
A) (17.4/22.4) (2/3) mol
B) (17.4/22.4) (3/2) mol
C) (17.4) (2/3) mol
D) (17.4/22.4) mol
E) (17.4) (22.4/3) mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q22: A gaseous mixture consists of 50.0% O<sub>2</sub>,25.0%
Q23: What is the ratio of the diffusion
Q24: Calculate the height of a column of
Q25: The energy of molecules of a gas:<br>A)is
Q26: If a liter of CO<sub>2</sub> is compared
Q28: In the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="In the
Q29: A 1.37 L vessel contains He at
Q30: According to the kinetic-molecular theory of gases,at
Q31: Under conditions for which chlorine gas has
Q32: In a sample of air at STP,the