Multiple Choice
In the reaction 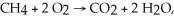
How many liters of O2 react with 5.0 liters of CH4? All gas volumes are measured at the same temperature and pressure but not at STP.
A) 0.22 L
B) 10 L
C) 5.0 L
D) 2.5 L
E) 20 L
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q23: What is the ratio of the diffusion
Q24: Calculate the height of a column of
Q25: The energy of molecules of a gas:<br>A)is
Q26: If a liter of CO<sub>2</sub> is compared
Q27: In the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="In the
Q29: A 1.37 L vessel contains He at
Q30: According to the kinetic-molecular theory of gases,at
Q31: Under conditions for which chlorine gas has
Q32: In a sample of air at STP,the
Q33: If 250.L of nitrogen reacts with 582