Multiple Choice
A gas of twice the molecular mass as CO2 will pass through a small hole how much faster than CO2?
A) 1/2 as fast
B) 2 times as fast
C) 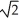 as fast
as fast
D) (1/ 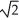 ) as fast
) as fast
E) 4 times as fast
Correct Answer:

Verified
Correct Answer:
Verified
Q56: According to the kinetic-molecular theory of gases:<br>A)gaseous
Q57: Gas densities depend strongly on gas temperature
Q58: If CO<sub>2</sub> and NH<sub>3</sub> are allowed to
Q59: The statement,"For a fixed mass of gas
Q60: A 34.8 mL sample of an unknown,water-insoluble
Q62: The statement,"For a fixed mass of gas
Q63: Chloroform (CHCl<sub>3</sub>)became popular as an anesthetic after
Q64: A 5.00 L container of unknown gas
Q65: What volume of acetylene gas,C<sub>2</sub>H<sub>2</sub>,would be required
Q66: Three identical flasks contain three different gases