Multiple Choice
Three identical flasks contain three different gases at standard temperature and pressure.Flask A contains 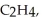 Flask B contains O3,and flask C contains F2.Which flask contains the largest number of molecules?
Flask B contains O3,and flask C contains F2.Which flask contains the largest number of molecules?
A) flask A
B) flask B
C) flask C
D) All contain the same number of molecules.
Correct Answer:

Verified
Correct Answer:
Verified
Q61: A gas of twice the molecular mass
Q62: The statement,"For a fixed mass of gas
Q63: Chloroform (CHCl<sub>3</sub>)became popular as an anesthetic after
Q64: A 5.00 L container of unknown gas
Q65: What volume of acetylene gas,C<sub>2</sub>H<sub>2</sub>,would be required
Q67: The volume in L H<sub>2</sub>(g)(measured at 22
Q68: How many liters of gas is 10.8
Q69: Which of the following is a characteristic
Q70: Of the following values,the most likely to
Q71: What is the temperature of NO<sub>2</sub> gas