Multiple Choice
Which statement is correct for the structure shown? 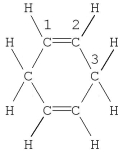
A) The molecule contains a total of 16 σ bonds.
B) Carbon no.1 is described by sp hybridization.
C) The molecule contains a total of four π bonds.
D) Carbon no.3 is described by sp3 hybridization.
E) The molecule contains a delocalized π bond system.
Correct Answer:

Verified
Correct Answer:
Verified
Q94: A triple bond is two sigma bonds
Q95: The hybrid orbital set used by the
Q96: The double covalent bond between two carbon
Q97: A double bond is two sigma bonds.
Q98: Using the VSEPR model,the electron-domain geometry of
Q99: The geometry of sp<sup>2</sup> hybridized orbitals is
Q101: What would be the bond order of
Q102: If the HCOO<sup>-</sup> ion is described using
Q103: Choose the INCORRECT statement about NH<sub>4</sub><sup>+</sup>.<br>A)The hybridization
Q104: The description of covalent bond formation as