Multiple Choice
The hybrid orbital set used by the central atom in NO3- is:
A) sp
B) 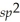
C) 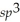
D) 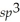 d
d
E) 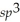
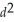
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q90: Which statement regarding VB theory is INCORRECT?<br>A)d
Q91: According to MO theory,which is the INCORRECT
Q92: The octahedral hybrid configuration is composed of
Q93: Molecular orbitals can be formed by adding
Q94: A triple bond is two sigma bonds
Q96: The double covalent bond between two carbon
Q97: A double bond is two sigma bonds.
Q98: Using the VSEPR model,the electron-domain geometry of
Q99: The geometry of sp<sup>2</sup> hybridized orbitals is
Q100: Which statement is correct for the structure