Multiple Choice
For the reaction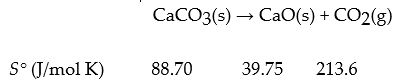
What is DrSo?
A) 262.5 J/mol ∙ K
B) -85.1 J/mol ∙ K
C) -164.7 J/mol ∙ K
D) 164.7 J/mol ∙ K
E) 85.1 J/mol ∙ K
Correct Answer:

Verified
Correct Answer:
Verified
Q83: Find correct statements.<br>I.A spontaneous process is a
Q84: The following reaction is exothermic.<br>2 N<sub>2</sub>O(g)→ 2
Q85: A reaction is spontaneous if:<br>I.ΔG is a
Q86: What is Δ<sub>r</sub>G°?<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="What is Δ<sub>r</sub>G°?
Q87: Which of the following has the highest
Q89: What is Δ<sub>r</sub>G° at 25 °C?<br>CO(g)+ 2
Q90: What is Δ<sub>r</sub>G°?<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="What is Δ<sub>r</sub>G°?
Q91: ?<sub>r</sub>G ? is independent of temperature.
Q92: Calculate Δ<sub>r</sub>G° for the reaction Cu(s)+ H<sub>2</sub>O(g)→
Q93: What is Δ<sub>r</sub>G° at 25 °C?<br>2O<sub>3</sub>(g)→ 3